Articles
- Page Path
- HOME > Sci Ed > Volume 7(2); 2020 > Article
-
Case Study
Analysis of consultations by the Committee for Publication Ethics of the Korean Association of Medical Journal Editors -
You Sun Kim1,2
 , Dong Soo Han1,3
, Dong Soo Han1,3
-
Science Editing 2020;7(2):184-188.
DOI: https://doi.org/10.6087/kcse.215
Published online: August 20, 2020
1Committee for Publication Ethics of the Korean Association of Medical Journal Editors, Seoul, Korea
2Department of Internal Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea
3Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- Correspondence to Dong Soo Han hands@hanyang.ac.kr
• Received: June 22, 2020 • Accepted: July 5, 2020
Copyright © 2020 Korean Council of Science Editors
This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article has been corrected. See "Declaration of conflict of interest for editorial board members’ articles" in Volume 9 on page 92.
Abstract
- This study aimed to analyze the inquiries on research and publication ethics submitted to the Committee for Publication Ethics of the Korean Association of Medical Journal Editors. A total of 80 inquiries were initiated over the course of 3 years, from April 2017 to March 2020. Based on a categorization of these inquiries, four common topics are discussed in detail. We present specific cases derived from actual situations, and the steps taken in processing these inquiries. The number of inquiries by topic was as follows: duplicate publications (12), secondary publications (11), authorship disputes (11), informed consent (6), proceedings (5), copyright (5), institutional review board approval (5), plagiarism (4), corrections (4), and others (17). Cases of duplicate publication and authorship disputes can be treated according to the flow chart of the Committee on Publication Ethics of the United Kingdom. Secondary publications may be permitted if the readers or audiences are different and both journals’ editors grant permission. Editors should be cautious about publishing cases without informed consent, even in the absence of identifiable photos, because patients or their families may be able to identify the cases. An adequate awareness of ethical considerations relevant to publication can help reduce the number of instances of research and publication ethics misconduct.
- Background/rationale: The importance of publication ethics cannot be overemphasized. To deal with questions and disputes among authors and/or editors, the Committee for Publication Ethics was established by the Korean Association of Medical Journal Editors (KAMJE) in 2006. The Committee receives inquiries from member societies and editors. Based on the seriousness of the inquiries, the Committee responds through official or informal deliberations. Nonetheless, we emphasize that the Committee is not a legal consultant and note that it was established to enhance the quality of medical journals.
- Objectives: We present several cases derived from actual situations and the steps followed in processing them. These cases were chosen to help editors, authors, and journals when they encounter ethical issues in the publication process. This study examines the most common and important consultations such as those on duplicate publications, secondary publications, authorship disputes, and informed consent. We believe that this study can help editors and authors by addressing their concerns.
Introduction
- Ethics statement: Neither institutional review board approval nor informed consent was required because this study is based on consultation reports.
- Study design: This is a descriptive and narrative study on the results of consultations during a 3-year period.
- Data collection and analysis: We analyzed the inquiries received by the Committee, which belongs to the KAMJE, between April 2017 and March 2020. Most inquiries came from the member societies of the KAMJE, and some minor inquiries came from individuals. Reviews and consultations on various aspects of publication ethics were requested in 80 inquiries, which we grouped according to the topics, and we reported the content of the deliberations conducted in response to the inquiries. Official deliberations were conducted through panel discussions with experienced ethics experts who were members of the Committee. Briefly, two members of the Committee were assigned to review each case, and they presented their opinions. Subsequently, all members of the Committee discussed the inquiry and gave their comments. Finally, the consensus opinions were circulated again and if there were no dissenting opinions, the content of the official deliberation was sent to the member societies. Informal deliberations were carried out by two experienced ethics experts of the Committee.
Methods
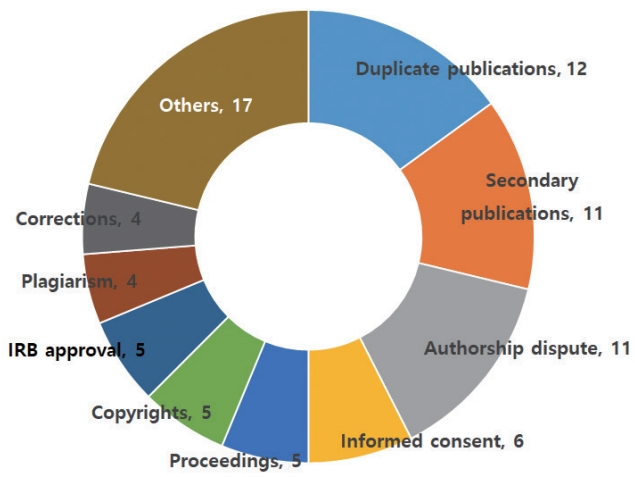
- Among the 80 inquiries, 13 were addressed through official deliberations and the remaining were handled through informal deliberations. These inquiries were categorized as dealing with duplicate publications (12), secondary publications (11), authorship disputes (11), informed consent (6), proceedings (5), copyright (5), institutional review board approval (5), plagiarism (4), corrections (4), and others (17) (Fig. 1).
- Duplicate publications
- Duplicate publications were the most common topic of consultations (15%). This term refers to the publication of an article that overlaps substantially with an earlier article published elsewhere without a proper citation [1]. Duplicate publication is a form of research misconduct and is prohibited because it wastes resources such as the review process and editor’s activity, as well as space in journals. It can cause results to be overestimated owing to an increase in the number of papers on a given subject without any substantive enhancements. Furthermore, duplicate publication can breach copyright [1].
- All suspected cases of duplicate publication were reviewed through official deliberations. In 2011, the Committee for Publication Ethics published sample cases of duplicate publications [2]. Here, we introduce an example that hints at the possibility of a duplicate publication. While reviewing a submitted manuscript, an editor searched for papers to determine its correspondence with earlier publications and found that the submitted manuscript was starkly similar to an earlier publication, in terms of both the topics chosen and the methods used. Several sentences were identical in the abstract, methods, and discussion sections of both papers. The similarity index showed an incredible rate of 86%. The editor asked for this case to be treated as a real instance of a duplicate publication and sought information on how this could be addressed.
- After an internal discussion, the Committee concluded that this was a case of duplicate publication by evaluating it against the established criteria [3]. Both papers had similar hypotheses, used identical methods, produced similar results, and involved an identical corresponding author and several co-authors. There was no new information in the subsequent paper. Duplicate publications are of three kinds: copy, salami, and imalas publications [4]. This case was classified as a salami publication. As several identical sentences were found, it was clear that text recycling had been carried out, which was a step too far. We recommended that the editor follow the Committee on Publication Ethics (COPE) flow chart [5], which requests the corresponding author to present an explanation. If this explanation is found inadequate, the editors are obliged to contact the co-authors of that paper and institutional leaders of the corresponding author, such as a department chair.
- Interestingly, duplicate publication was the most common reason (57.0%) for retraction in 111 papers that were published and retracted in KoreaMed from 1990 to January 2016 [6]. This result is markedly different from Western studies, which reported that around 15.8% to 17% of retractions were due to duplicate publication [7,8]. Some papers were retracted inappropriately, such as retraction of the first article published in a case of duplicate publication. This result may be associated with the recent publication awareness campaign in Korea to prevent duplicate publication [9]. In recent years, editors have been recommended to use a similarity check system when they receive a paper submission to help detect possible plagiarism and duplicate publication [10]. Altogether, duplicate publication is an important issue in publication ethics and should be prevented.
- Secondary publications
- Many editors had questions about secondary publications. Editors reported having occasionally received requests from certain societies or institutes to publish a commentary or a mini-review of public health issues in different journals. The editors wanted to know if doing so would lead to a duplicate publication problem and accordingly, how this could best be addressed. The term “secondary publication” is defined as a permitted duplicate publication that meets established criteria [1]. Several conditions need to be fulfilled for a secondary publication: the permission of editors of both journals must be sought, both journals should have different reader groups and audiences, the previous publication should be named in a footnote (“This article is based on a study first reported in the < Journal title > , < full reference > ”), and the article must have a title that indicates the paper has been published as a secondary publication (republication, summary, etc.) [1]. Secondary publications can be simultaneous or joint. According to the International Committee of Medical Journal Editors (ICMJE) guidelines, in cases of a public health emergency, duplicate submissions and publications may be permitted. The important consideration is that the editors of both journals should be notified in advance. Editors should also check the conditions for secondary publication and mention the secondary publication in a footnote.
- Authorship dispute
- Being an author of a scientific manuscript is a privilege and an honor for a scientist. Authorship represents a critical element of scientific research and conveys professional benefits and responsibilities. However, authorship is one of the most commonly disputed areas. The Committee received several inquiries about authorship. The most common inquiries dealt with adding or deleting a specific author or authors to and from already published articles.
- The ICMJE guidelines provided criteria for updated authorship in 2013 and indicated that individuals listed as authors must satisfy all four criteria [1]: “1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; 2) Drafting the work or revising it critically for important intellectual content; 3) Final approval of the version to be published; and 4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.” An individual who does not meet all four criteria should be mentioned in the acknowledgments or contributorship section, rather than as an author. However, authorship abuse can occur and takes several forms, including coercive authorship, honorary or gift authorships, and ghost authorship [11,12]. In an authorship dispute involving the deletion or addition of specific authors, we recommend that if there is a consensus among all authors to add or delete a specific author or authors and if they are able to provide a suitable reason to the editor for doing so, a change in authorship can be made according to the COPE flow chart [5]. A correction letter should then be issued. It is important to note that author disputes are not the responsibility of editors or journals. This issue should be resolved among the authors themselves and institutions should step in only if these problems persist.
- There have been concerns about inappropriate authorship in Korea because the number of authors in original articles from a single institution in Korea is larger than that of other countries. It is recommended that Korean researchers be aware of and follow the global standards of publication ethics regarding authorship [13].
- Informed consent
- Informed consent involves securing permission to disclose personal information in research. It is gaining more importance in the publication process, and journals are strongly recommended to protect the personal information of the patients that are presented in the articles they publish. The General Data Protection Regulation (GDPR) implemented by the European Union aims to protect the personal data of individuals [14]. According to the GDPR, without prior informed consent, no personal information, including pictures, can be published in journals. The authors should obtain informed consent from their study subjects and clarify and confirm the extent to which their information will be exposed in a manuscript before publication. The Committee received several inquiries about informed consent. In one case, a child had a very rare disease, but the parents refused permission to report the case. Therefore, the author omitted photographs showing the child’s face and other pictures in which the child was recognizable. The authors stated that they did not obtain the permission of the parents and thus omitted the pictures. However, the editor was concerned about the publication of this report because even though there were no personal data, the authors did not have permission to present the relevant information. Thus, the Committee responded by saying that although the case had academic value, without the permission of the parents of the child, it was unethical to publish the report, especially as it was likely to encounter major problems after publication. Editors are expected to check the personal data protection strategy and the acquisition of informed consent in the course of processing and evaluating submissions to the journal. Authors should present details about how informed consent was obtained from subjects in their manuscripts.
Results
- Ethical issues in publication are more important now than ever before. According to the “Regulation on the management of national research and development” by the Korean government, research misconduct includes fabrication, falsification, plagiarism, inappropriate authorship, and duplicate publication (https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=43461&type=sogan&key=54). Among them, inappropriate authorship and duplicate publication involve misconduct of publication ethics. By explaining some cases addressed by the Committee, we believe that a heightened awareness of particular ethical challenges that are relevant to academic publishing can help authors, reviewers, and editors reduce instances of misconduct. In addition, we recommend referring to the third edition of the Good publication practice guideline for medical journals by the Committee for Publication Ethics [15].
Conclusion
Fig. 1.Categorization and distribution of 80 inquiries on research and publication ethics to the Committee for Publication Ethics of the Korean Association of Medical Journal Editors from April 2017 to March 2020. IRB, institutional
review board.

- 1. International Committee of Medical Journal of Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals [Internet]. International Committee of Medical Journal of Editors. 2019 [cited 2020 Apr 22]. Available from: http://www.icmje.org/recommendations/.
- 2. Bae CW, Kim SY, Huh S, Hahm CK. Sample cases of duplicate publication. Seoul: Korean Association of Medical Journal Editors; 2011 https://doi.org/10.5082/duplicate_publication.2011.7. Article
- 3. Cho BK, Rosenfeldt F, Turina MI, et al. Joint statement on redundant (duplicate) publication by the editors of the undersigned cardiothoracic journals. Ann Thorac Surg 2000;69:66. https://doi.org/10.1016/s0003-4975(00)01088-2. ArticlePubMed
- 4. von Elm E, Poglia G, Walder B, Tramer MR. Different patterns of duplicate publication: an analysis of articles used in systematic reviews. JAMA 2004;291:974-80.https://doi.org/10.1001/jama.291.8.974. ArticlePubMed
- 5. Committee on Publication Ethics. Flowcharts [Internet]. London: Committee on Publication Ethics; [cited 2020 Apr 22]. Available from: http://publicationethics.org/resources/flowcharts.
- 6. Huh S, Kim SY, Cho HM. Characteristics of retraction from Korean medical journals in the KoreaMed database: a bibliometric analysis. Plos One 2016;11:e0163588. https://doi.org/10.1371/journal.pone.0163588. ArticlePubMedPMC
- 7. Steen RG. Retractions in the scientific literature: is the incidence of research fraud increasing? J Med Ethics 2011;37:249-53.https://doi.org/10.1136/jme.2010.040923. ArticlePubMed
- 8. Wager E, Williams P. Why and how do journals retract articles? An analysis of Medline retractions 1988-200 J Med Ethics 2011;37:567-70.https://doi.org/10.1136/jme.2010.040964. ArticlePubMed
- 9. Kim SY, Bae CW, Hahm CK, Cho HM. Duplicate publication rate decline in Korean medical journals. J Korean Med Sci 2014;29:172-5.https://doi.org/10.3346/jkms.2014.22.172. ArticlePubMedPMC
- 10. Choi J, Park S, Oh U. CrossCheck usage in a journal publication. Sci Ed 2016;3:26-32.https://doi.org/6087/kcse.59. ArticlePDF
- 11. Strange K. Authorship: why not just toss a coin? Am J Physiol Cell Physiol 2008;295:C567-75.https://doi.org/10.1152/ajpcell.00208.2008. ArticlePubMedPMC
- 12. Claxton LD. Scientific authorship. Part 2. History, recurring issues, practices, and guidelines. Mutat Res 2005;589:31-45.https://doi.org/10.1016/j.mrrev.2004.07.002. ArticlePubMed
- 13. Hong ST. Avoiding inappropriate authorship. J Korean Med Sci 2017;32:1046-7.https://doi.org/10.3346/jkms.2017.32.6.1046. ArticlePubMedPMC
- 14. European Parliament and the Council of the European Union. General Data Protection Regulation. Document 32016R0679 (27 April, 2016) [Internet]. Luxembourg: EUR-Lex; [cited 2020 Apr 22]. Available from: https://eur-lex.europa.eu/eli/reg/2016/679/oj.
- 15. In : Han DS, editor. Good publication practice guideline for medical journals by the Committee for Publication Ethics. 3rd ed. Seoul: Korean Association of Medical Journal Editors; 2019.
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Ethics Committees: Structure, Roles, and Issues
Pankti Mehta, Olena Zimba, Armen Yuri Gasparyan, Birzhan Seiil, Marlen Yessirkepov
Journal of Korean Medical Science.2023;[Epub] CrossRef - Analysis of duplicated publications in Russian journals
Yury V. Chekhovich, Andrey V. Khazov
Journal of Informetrics.2022; 16(1): 101246. CrossRef - Consultation questions on publication ethics from 2016 to 2020 addressed by the Committee on Publication Ethics of the Korean Council of Science Editors
Woo Jin Son, Cheol-Heui Yun
Science Editing.2021; 8(1): 112. CrossRef

 KCSE
KCSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite